Use Case
TrialPulse — DCT Design and Informed Consent
Case Study
Leveraging the Patient Voice to Collect Parkinson’s Disease Patient and Caregiver Input on a DCT Informed Consent document
Client Goal
In preparation for a soon-to-launch decentralized clinical trial (DCT), our client wished to understand how participants react to and interpret the Informed Consent, particularly regarding the Study Purpose, Participant Responsibilities, and Potential Risks & Benefits.
Objective 1
Readability: How clear and accessible do participants find the Informed Consent document?
Objective 2
Red flags: What questions or concerns does the Informed Consent document raise for trial participation?
Objective 3
Raising the bar: How could the Informed Consent document be improved or optimized to better support DCT recruitment and retention?
The inVibe Solution
inVibe’s TrialPulse solution allowed our pharmaceutical client to quickly and efficiently deploy a voice-powered research study to validate and optimize the Informed Consent document.
1. Collect
Within 48 hours, inVibe screened and recruited 20 patients (and their loved ones) who were diagnosed with Parkinson’s Disease across different stages of their journey. The participants participated in an automated phone interview on their computers or smart phones, responding to questions by speaking.
Key Questions
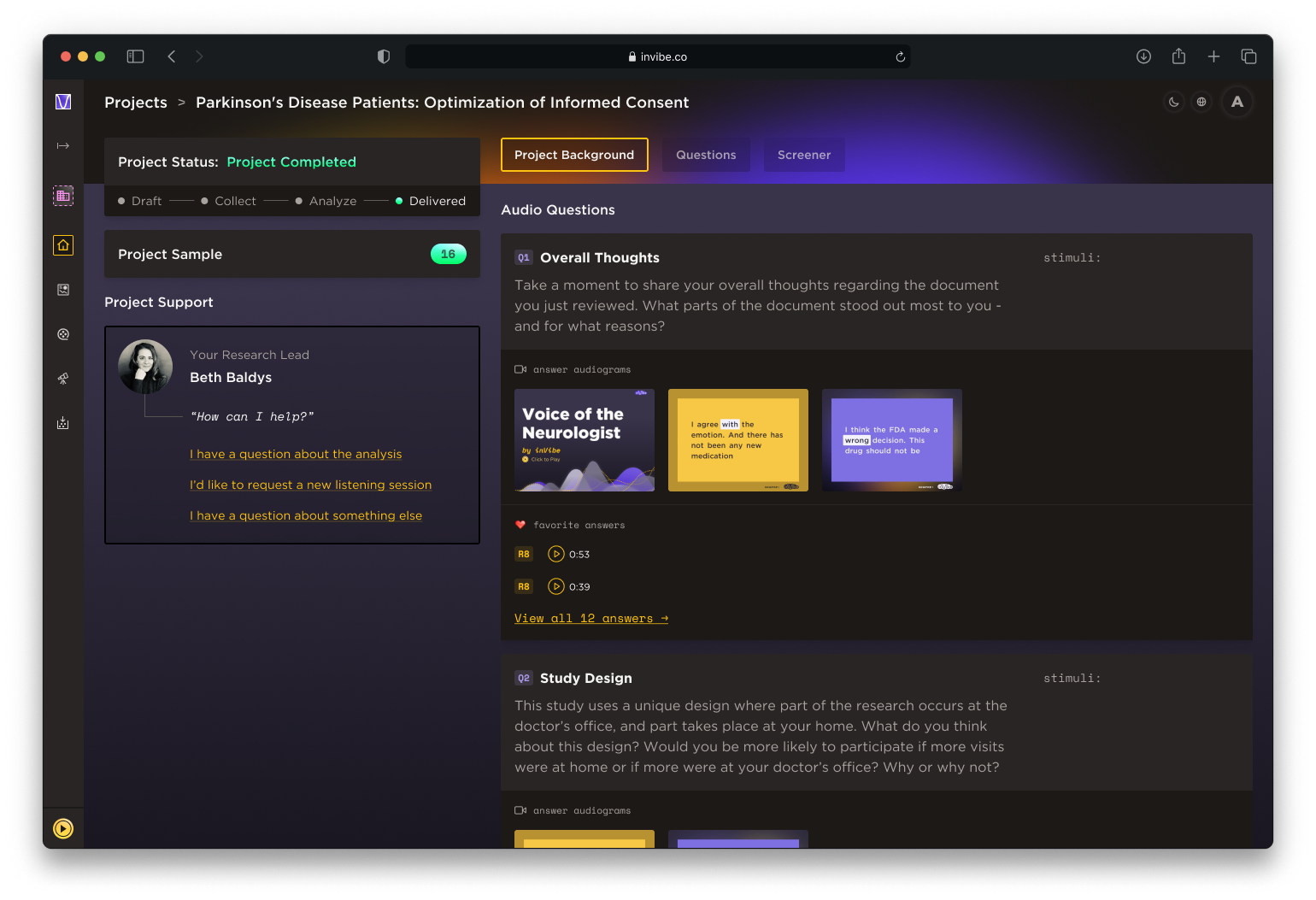
Q1
Take a moment to share your overall thoughts regarding the document you just reviewed. What parts of the document stood out most to you – and for what reasons?
Q2
This study uses a unique design where part of the research occurs at the doctor’s office, and part takes place at your home. What do you think about this design? Would you be more likely to participate if more visits were at home or if more were at your doctor’s office? Why or why not?
Q3
The study asks you to wear a wrist device and use a tablet to complete certain study requirements at home. Do you have concerns about this portion of the study? And if so, what could improve the at-home parts of the research?
Q4
Let’s talk about the section regarding potential risks. Based on what you reviewed in the document, what are the biggest potential risks for participating in this trial? Do you see any of these risks as dealbreakers? Why or why not?
2. Analyze
inVibe’s analysts leveraged their language expertise and advanced NLP tools to process, evaluate and analyze multiple aspects of each response, including content, language, and emotion derived from speech-emotion recognition, allowing for deeper insights, faster.
What is Said
Summarize and categorize the content and themes.
How it’s Said
Identify the language patterns and construction.
How it Sounds
Utilize machine learning and acoustic technology to assess the emotionality.
3. Deliver
The client was provided with an interactive report via an online dashboard, along with the voice data, transcripts, and a high-level analysis. An in-house analyst walked the client through the most meaningful data, supporting each insight with specific voice data.
Audiogram
Videos
Clients have access to drag & drop tools to instantaneously create kinetic text animations from transcripts for internal sharing.

Listening
Labs
inVibe delivers self-guided interactive stories assembled from transcripts, audio, and charts – annotated with high-level expert observations.

Expert
Perspective
inVibe’s team of in-house analysts and language experts walk clients through the insights and recommendations derived from the research.
Key Insights & Recommendations
Based on the insights, inVibe recommended that the team take the following actions to optimize their Informed Consent document:
Side effects
#1
Action:
Patients expressed concern about the lack of details related to potential side-effects. This led them to make assumptions, which caused additional worry. inVibe recommended simple formatting and data visualization revisions to the Potential Risks and Discomforts section in order to simplify and clarify.
Decentralized design
#2
Action:
Most patients felt the study’s hybrid design strikes the right balance of in-home and in-office visits for this patient population. inVibe recommended including references to technical support for digital aspects of the study in order to address minor concerns.
Risks of participation
#3
Action:
The trial requirement for lumbar punctures was a red flag for some patients, who expressed confusion as to how such a procedure related to Parkinson’s. inVibe recommended explaining the diagnostic function of lumbar puncture procedures throughout the study to assuage concerns.
Ready to learn more?
/voice
/resources
/use-cases
- New Data Intelligence
- TrialPulse — Evaluation of Clinical Trial Recruitment Assets
- Concept / Message Testing
- Customer Journey Inflection Points
- Agency Pitch for New Business
- TrialPulse — DCT Design and Informed Consent
- Language of Disease
- Customer Stories
- Decision Drivers
- Market Trends
- TrialPulse — Disease Burden and Endpoint Selection
- HCP – Patient Communication
2025 inVibe - All Rights Reserved
